6´-Aminocinchonidine (CAS-No. 2143936-34-7) is a new member of the Cinchona Alkaloid family. The additional primary...
Buchler Glossary
Reformatsky reaction is an organic reaction which condenses aldehydes or ketones with α-halo esters using metallic zinc to form β-hydroxy-esters.
Reformatsky reaction examples catalyzed by cinchona alkaloid derivatives can be found in our free of charge Chiral Catalyst Search Data Base.
Example from Literature
Highly Enantioselective Reformatsky Reaction of Ketones: Chelation-Assisted Enantioface Discrimination. (Ojida et al.; Org. Lett. 2002, 4, 3051-3054.)
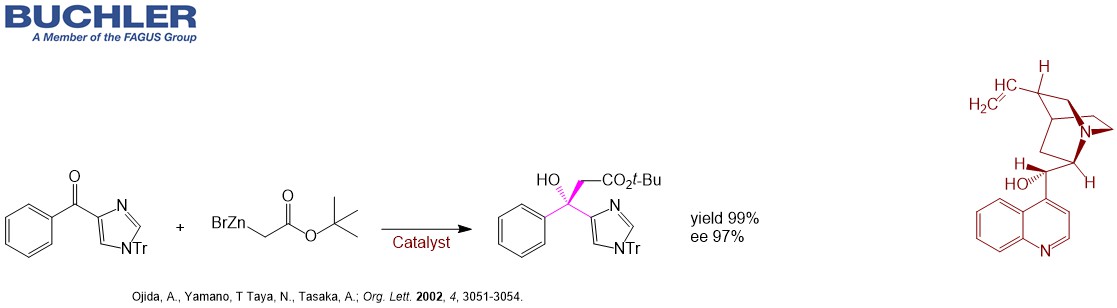
Further Articles:
6´-Aminodihydrocinchonidine (CAS-No. 107781-90-8)
6´-Aminodihydrocinchonidine Base (CAS-No. 107781-90-8) is a cinchona alkaloid closely related to 6´-Aminocinchonidine....
6´-Aminodihydrocinchonine (CAS-No. 911708-35-5)
6´-Aminodihydrocinchonine Base (CAS-No. 911708-35-5) is a cinchona alkaloid closely related to 6´-Aminocinchonine....
Alkynylation
Alkynylation is an addition reaction in organic synthesis where a terminal alkyne adds to a carbonyl group to form an...
Allylsilylations
Allylsilylations are performed between allylsilanes and aldehydes furnishes homo allyl alcohols. Using Cinchona...
Aminohydroxylation
The Sharpless Aminohydroxylation allows the syn-selective preparation of 1,2-amino alcohols by reaction of alkenes...
