Any hydrocarbon or heterocycle with 4n+2 electrons in a fully conjugated cyclic π system is considered to be an...
Buchler Glossary
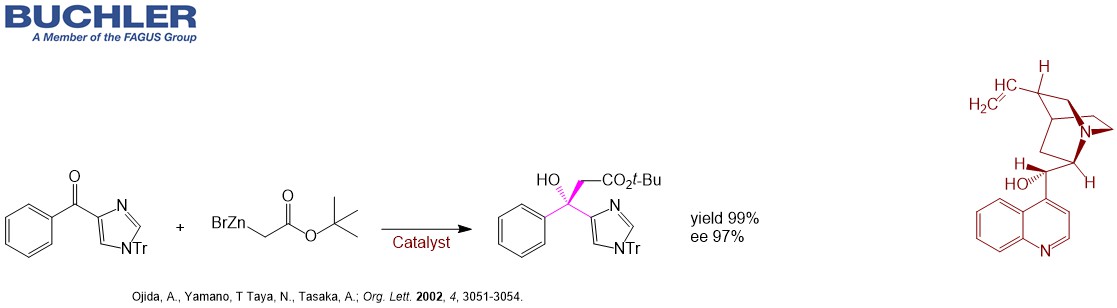
Reformatsky reaction is an organic reaction which condenses aldehydes or ketones with α-halo esters using metallic zinc to form β-hydroxy-esters.
Reformatsky reaction examples catalyzed by cinchona alkaloid derivatives can be found in our free of charge Chiral Catalyst Search Data Base.
Example from Literature
Highly Enantioselective Reformatsky Reaction of Ketones: Chelation-Assisted Enantioface Discrimination. (Ojida et al.; Org. Lett. 2002, 4, 3051-3054.)
Further Articles:
Asymmetric Aldol Reaction
The aldol reaction is one of the most important and effective methodologies for the straightforward construction of...
Asymmetric Alkylation
Alkylation is the introduction of an alkyl group into an organic compound by substitution or addition. The alkyl group...
Asymmetric Amination
Amination is the process by which an amine group is introduced into an organic molecule. This type of reaction is...
Asymmetric Dihydroxylation
Asymmetric Dihydroxylation of olefins is of high synthetic value because it introduces two vicinal hydroxy groups,...
