Asymmetric oxohydroxylation of α-alkyl enoates with potassium permanganate catalyzed by monocationic quaternary...
Buchler Glossary
Reformatsky reaction is an organic reaction which condenses aldehydes or ketones with α-halo esters using metallic zinc to form β-hydroxy-esters.
Reformatsky reaction examples catalyzed by cinchona alkaloid derivatives can be found in our free of charge Chiral Catalyst Search Data Base.
Example from Literature
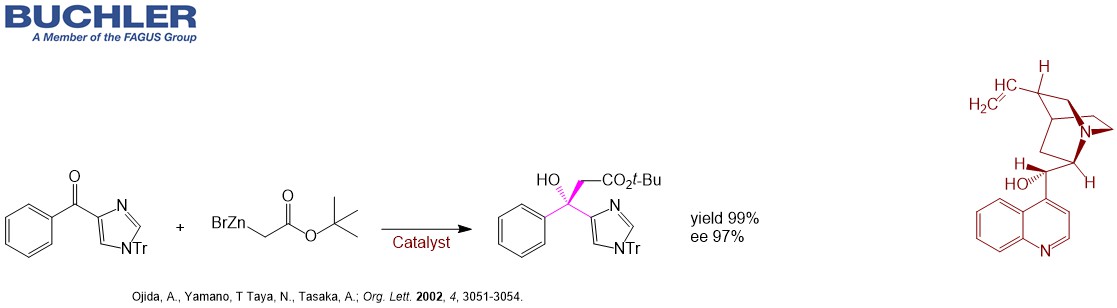
Highly Enantioselective Reformatsky Reaction of Ketones: Chelation-Assisted Enantioface Discrimination. (Ojida et al.; Org. Lett. 2002, 4, 3051-3054.)
Further Articles:
(DHQ)2PHAL (CAS-No. 140924-50-1)
(DHQ)2PHAL - Dihydroquinine 1,4-phthalazinediyl diether (CAS-No. 140924-50-1) The well-known Cinchona alkaloid...
(DHQD)2PHAL (CAS-No. 140853-10-7)
(DHQD)2PHAL - Dihydroquinidine 1,4-phthalazinediyl diether (CAS-No. 140853-10-7) The well-known Cinchona alkaloid...
1,2-Addition
1,2-Addition is a type of organic chemical reaction that involves the addition of functional groups to the 1st and 2nd...
1,4-Addition (conjugate addition)
The Michael reaction is the conjugate 1,4-Addition of a resonance stabilized carbanion (michael donor) to an activated...
6`-Aminocinchonine (CAS-No. 2143936-31-4)
6´-Aminocinchonine Base (CAS-No. 2143936-31-4), a pseudoenantiomer of 6´-Aminocinchonidine, has the same...
