Asymmetric oxohydroxylation of α-alkyl enoates with potassium permanganate catalyzed by monocationic quaternary...
Buchler Glossary
The Henry reaction, also called Nitroaldol reaction, is a classic carbon–carbon bond formation reaction in organic chemistry. This kind of reaction is a base-catalyzed C-C bond-forming reaction between nitroalkanes and aldehydes or ketones. Because of its similarity with the aldol reaction it is also referred as the nitroaldol reaction. The Henry reaction of ketones has become one of the most important and versatile reactions for the construction of quaternary carbons containing hydroxyl and nitro groups.
The asymmetric Henry reaction can be accomplished by using a huge variety of Cinchona alkaloid based catalysts.
Asymmetric Henry reaction
A significant feature of the Henry reaction is a possibility to conduct the reaction enantio- or diastereoselectively using easily accessible Cinchona alkaloid catalysts. Chiral products of the Henry reaction, 2-nitroalcohols, are important intermediates in syntheses of many biologically active compounds. The core structure of the Cinchona alkaloid serves as an efficient enantioselective organocatalyst. Both stereoisomers can easily be synthesized enantioselectively using an organocatalyst from the blue or red Cinchona alkaloid series. The asymmetric Henry reaction represents a powerful carbon-carbon bond-forming procedure for the preparation of valuable synthetic intermediates. The nitro alcohols can be further transformed in a number of important chiral amino alcohols.
Please review lots of examples for asymmetric Henry reactions in our free of charge Chiral Catalyst Search Data Base.
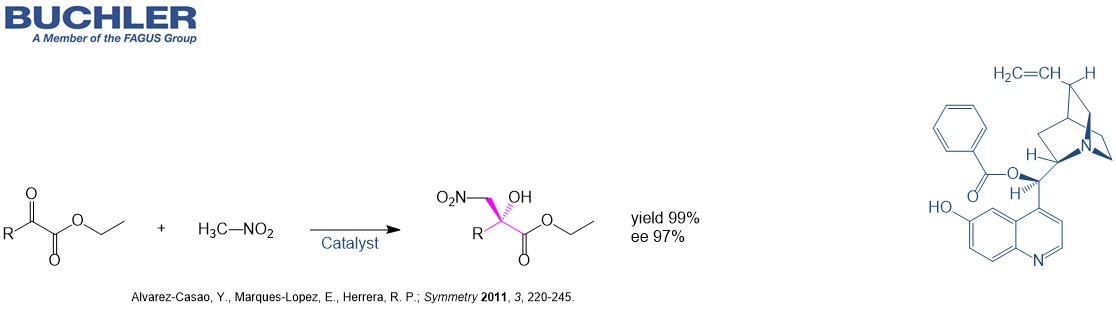
Example from Literature
Organocatalytic Enantioselective Henry Reactions. (Alvarez-Casao et al.; Symmetry 2011, 3, 220-245.)
Further Articles:
(DHQ)2PHAL (CAS-No. 140924-50-1)
(DHQ)2PHAL - Dihydroquinine 1,4-phthalazinediyl diether (CAS-No. 140924-50-1) The well-known Cinchona alkaloid...
(DHQD)2PHAL (CAS-No. 140853-10-7)
(DHQD)2PHAL - Dihydroquinidine 1,4-phthalazinediyl diether (CAS-No. 140853-10-7) The well-known Cinchona alkaloid...
1,2-Addition
1,2-Addition is a type of organic chemical reaction that involves the addition of functional groups to the 1st and 2nd...
1,4-Addition (conjugate addition)
The Michael reaction is the conjugate 1,4-Addition of a resonance stabilized carbanion (michael donor) to an activated...
6`-Aminocinchonine (CAS-No. 2143936-31-4)
6´-Aminocinchonine Base (CAS-No. 2143936-31-4), a pseudoenantiomer of 6´-Aminocinchonidine, has the same...
