Product Data Sheet
Safety Data Sheet
Introduction
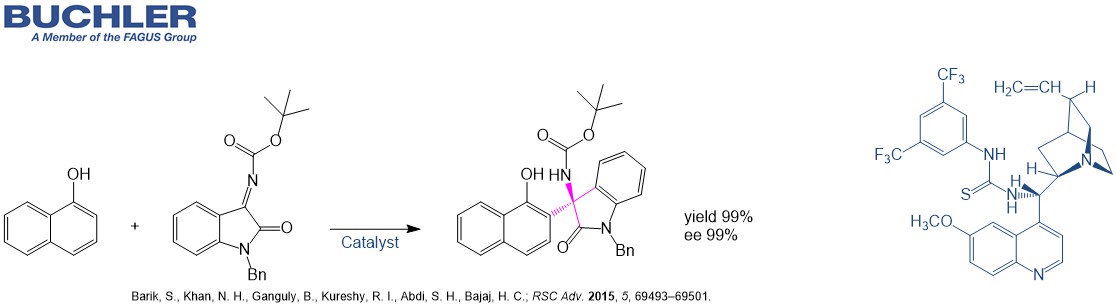
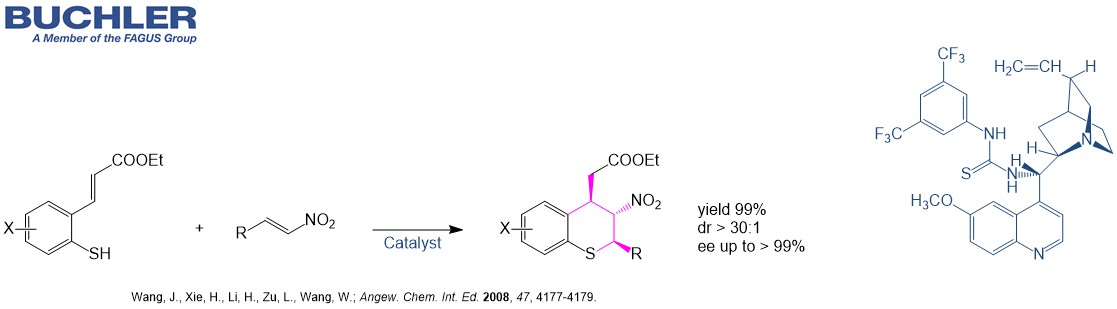
Cinchona Thiourea catalysts like the Quinine based dimer N-[3,5-Bis(trifluoromethyl)phenyl]-N′-[(8α,9S)-6′-methoxycinchonan-9-yl]thiourea (CAS-No. 852913-16-7) as well as the Quinidine based dimer N-[3,5-Bis(trifluoromethyl)phenyl]-N′-[(9R)-6′-methoxycinchonan-9-yl]thiourea (CAS-No. 852913-25-8) have been shown to be highly efficient and broadly applicable chiral catalysts for asymmetric reactions.The desired products can be afforded in high yields with good to excellent enantioselectivities. Typical applications of the Quinine based chiral Cinchona Thiourea catalysts are for example: addition, aldol, atropisomer synthesis, cyclization, cycloaddition, desymmetrization, Friedel-crafts, Henry, Mannich, Michael, spiroycylization and Strecker synthesis.
Several chiral application examples can be reviewed in our free of charge Chiral Catalyst Search Data Base.
Within organocatalysis, bifunctional Cinchona Thiourea catalysts have become one of the most important classes of highly efficient catalysts for a wide range of applications. Bifunctionality of these Cinchona Thiourea catalysts mean the capability to activate the nucleophile and electrophile reactants simultaneously. The Cinchona framework is one of the privileged chiral skeletons in asymmetric organocatalysis. The tertiary amino group in the quinuclidine ring gives basic character to the molecule, hence this can activate/fix nucleophiles or electrophiles, and is responsible for chiral induction. Connecting the cinchona skeleton to a double hydrogen-bond donor moiety like the thiourea, which can fix the corresponding substrate by forming a strong double hydrogen bond, extends their applicability.
Cinchona Thiourea catalysts are robust and tunable bifunctional organocatalysts and therefore synthetically useful for a wide range of transformations.
Physical properties
N-[3,5-Bis(trifluoromethyl)phenyl]-N′-[(8α,9S)-6′-methoxycinchonan-9-yl]thiourea is a white to beige crystalline powder. The melting point is 121 °C.
Synonyms
1-(3,5-Bis(trifluoromethyl)phenyl)-3-((1S)-(6-methoxyquinolin-4-yl) ((2R)-8-vinylquinuclidin-2-yl)methyl) thiourea and 1-(3,5-bis(trifluoromethyl)phenyl)-3-((S)-(6-methoxyquinolin-4-yl)((1S,2S,4S,5R)-5-vinylquinuclidin-2-yl)methyl)thiourea.
Associated Reactions
136 reactions found.
But only 10 are listed.
Please login below to see more.