Product Data Sheet
Safety Data Sheet
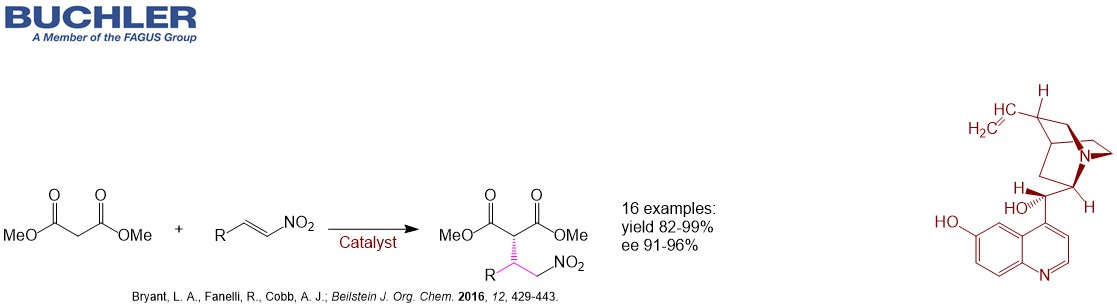
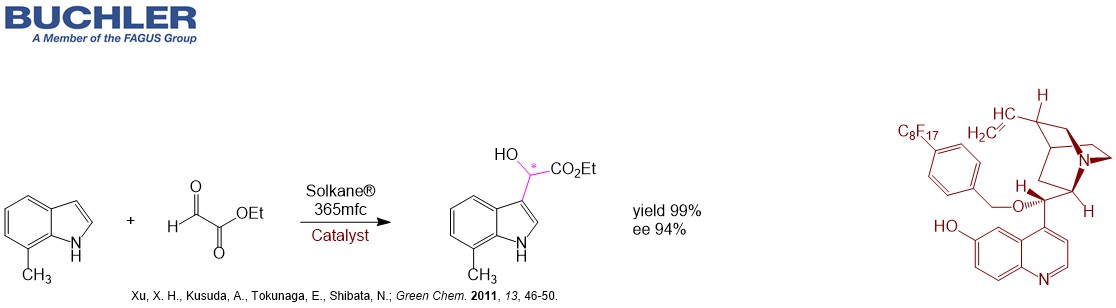
Cupreidine Base (CAS-No. 70877-75-7) as well as the pseudoenantiomer Cupreine belong to an emerging class of bifunctional highly enantioselective organocatalysts. They can be used for a wide range of asymmetric reactions.Similar to the amino group of 6´-Aminocinchonine the hydroxy group proves crucial to obtain high enantioselectivity in many synthetically relevant reactions like Michael additions, Henry, Diels-Alder, Friedel-Crafts, Fluorination, Amination, Alkylation, Bromolactonization etc. despite its position far away from the stereocenters. Usually the electrophile is activated via the C6`-hydroxy group. On the other hand the free C6’ hydroxy group can be further functionalized to improve catalytic performance. The key step of Indoxacarb is a α-hydroxylation of a β-ketoester. Higher enantiomeric excesses can be obtained with Cupreidine compared to Cinchonine. Cupreidine Base as a derivative of Quinidine is also widely used as a racemic resolution agent.For more detailed information please enter into our free of charge chiral catalyst database.
Physical properties
Cupreidine Base (CAS-No.: 70877-75-7) is a beige to pale yellow, light yellow powder soluble in DMSO, ethanol and methanol. The melting point is in the range of 184-190 °C. This compound is not hygroscopic and contains less than 5.0 % water. Cupreine could be stored at ambient conditions in well-closed, light-resistant containers.
Synonyms
O-Demethylquinidine, Desmethylquinidine 6`-Hydroxycinchonine, (8R,9S)-6`-Hydroxycinchonan-9-ol, (8β,9S)-6`-Hydroxycinchonan-9-ol and (8R,9S)-Cinchonan-6`,9-diol
NMR-data
The following spectroscopic techniques were used to provide structural evidence for Cupreidine Base by NMR technology: 1H NMR, 13C NMR, 1H-1H COSY, 1H-13C HSQC, 13C-1H HMBC, 1H-1H NOESY, 15N-1H HMBC.
NMR spectra of the structural elucidation can be viewed here:
NMR – Cupreidine BaseAssociated Reactions
59 reactions found.
But only 10 are listed.
Please login below to see more.