Product Data Sheet
Safety Data Sheet
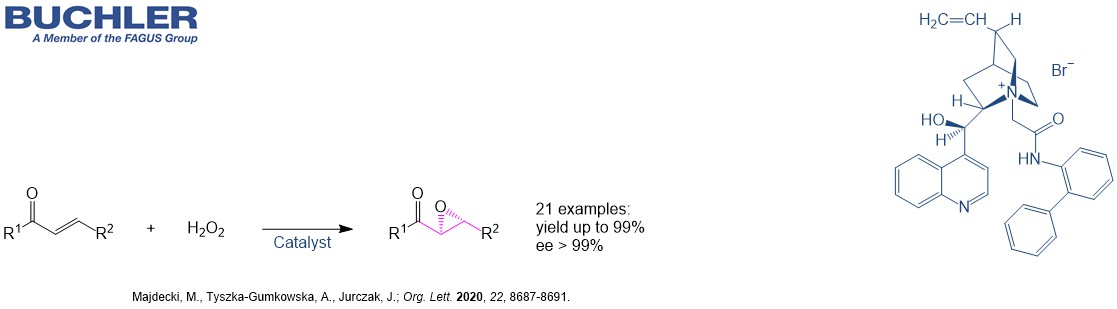
Cinchonidine Base (CAS-No. 485-71-2) as a natural product is extracetd from the bark of the cinchona tree. This compound has the same stereochemistry as the most famous Cinchona alkaloid Quinine. It is widely used in asymmetric Synthesis and racemic resolution processes. The demand of chiral compound has grown exponentially over the last two decades. The development of new enantioselective processes is highly relevant in chemistry due to the relevance of chiral compounds in pharamceutical, agrochemical and fine chemical industry. Nowadays, the term asymmetric organocatalysis covers a wide range of organic processes and methodologies, providing efficient and environmentally friendly access to enantiomerically pure compounds. Cinchonidine is a versatile tool in applied Green Chemistry.
Lots of highly enantioselective reactions promoted by Cinchonidine can be reviewed in our free of charge chiral catalyst search.
Physical properties
Cinchonidine Base (CAS-No.: 485-71-2) is a white or almost white, crystalline powder or it consists of fine, colourless needles, being soluble in ethanol, CHCl3 and methanol. The melting point is in the range of 200-207 °C. This compound is not hygroscopic and contains less than 1.0 % water. Cinchonidine could be stored at ambient conditions in well-closed, light-resistant containers.
Synonyms
Cinchovatine, (8S,9R)-Cinchonan-9-ol, (8α,9R)-Cinchonan-9-ol and (R)-[(2S,4S,5R)-5-Ethenyl-1-azabicyclo[2.2.2]oct-2-yl](quinolin-4-yl)methanol
Associated Reactions
142 reactions found.
But only 3 are listed.
Please login below to see more.